Research Group Developmental Neurobiology
Silvia Cappello
Neuronal migration is a fundamental step in the development of the central nervous system (Figure 1). Malformations of the human neocortex due to defects in neuronal positioning are present in >1% of the general population and represent a major cause of developmental disabilities and severe epilepsies. In order to understand the biological mechanisms and therefore identify potential therapies for cortical malformations, we need to first discover the regulation of all processes of cortical development. We therefore need mouse models that mimic the human disorders to be available and an in vitro/in vivo approach that enables us to translate the finding into human cells/brain. Once we know whether the cellular and molecular mechanisms are maintained between the two species, we can look at the circuitry and connectivity and identify the common regulators. We can also seek candidate molecules to use for potential therapy.
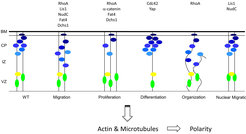
Fig. 2: Defects that lead to cortical malformations.
Gene mutations associated with defects in migration, proliferation, differentiation or organization of neural stem cells or migrating neurons.
VZ: ventricular zone, IZ: intermediate zone, CP: cortical plate, BM: basement membrane
In order to achieve this we want to identify novel genes responsible for cortical malformations (Figure 2) by mapping the genome of patients that do not have mutations in the ‘usual suspect’ genes (in collaboration with Dr. Stephen Robertson - human geneticist, University of Otago, New Zealand). We can then generate mouse models to investigate the molecular and cellular mechanisms underlying these diseases by gain and loss of function (Figure 3).
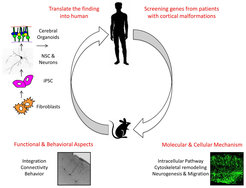
Fig. 3: Schematic drawing of the research plan.
The schema shows: 1) Screening of new candidate genes from human patients affected by cortical malformations, 2) and 3) modeling molecular, cellular and functional aspects of the malformations in the mouse model, 4) translation of the finding into humans by using human induced pluripotent stem cells (IPSC) derived neural stem cells, neurons and cerebral organoids.
Furthermore, we will characterize the functional aspects of these disorders by using our mouse models of cortical malformations (Cappello et al., Neuron 2012; Cappello et al., Nature Genetics 2013; Schimd et al., Frontiers in Neuroscience 2014) to address long-standing questions that are very difficult to directly address in human patients: how connectivity between heterotopias is established and what is the neuronal composition as well as behavior and electrophysiological properties of the ectopic neurons. Finally we will study the molecular, cellular and functional properties of human reprogrammed neural stem cells and neurons derived from patients and generate cerebral organoids to mimic the human brain (Figure 4).
In this way, we will be able to screen for several candidate genes identified from human patients as well as from known mouse models and assemble a network of genes and possible pathways responsible for cortical malformations.
With these methods, we will be able to significantly contribute towards the identification of strategies for therapeutic approaches, like for instance the re-expression of mutant genes or modulation of cytoskeleton stability. We also hope to develop a genetic test to screen for susceptibility, e.g. for epilepsy, at a very early stage of development.
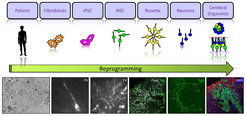
Fig. 4: Reprogramming human cells.
Upper panel: Schematic drawing of the different steps of reprogramming, starting from human fibroblasts of patients affected by cortical malformations that are sequentially reprogrammed in neural stem cells and neurons (in vitro 2D) or in cerebral organoids (in vitro 3D).
Lower panels: Examples of reprogrammed cells, IPSC, neural stem cells that are positive for phospho vimentin (PV) and Pax6, neurons positive for Tuj1.
Performing RNA sequencing and proteomics of different populations of human cells (e.g. neural stem cells versus neurons, or control versus mutant reprogrammed neurons) will ultimately enable us to define gene networks and pathways involved. This approach together with an appropriate functional analysis of the mouse models (e.g. functional MRI) will give us new ideas in order to discover new targets for drug therapies.


