Research Group Stress Resilience
Mathias Schmidt
Stress is part of everyday life. Everybody is exposed to stressful experiences and the body's stress response is adaptive and helps to deal successfully with stressful situations. So why is stress also a major risk factor for psychiatric and metabolic disorders? And why are some people resilient to stress, while others are vulnerable and become sick? These are the questions the Schmidt Lab is aiming to unravel.
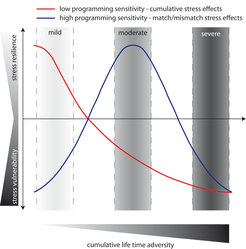
Effects of different degrees of life time adversity on stress vulnerability and resilience in adulthood in dependence of genetic predisposition.
An individual with a lower sensitivity to its early environment (red line) would therefore benefit most from life histories with a mild overall adversity, while each experienced adverse life event would increase subsequent stress vulnerability and disease probability (e.a. cumulative stress hypothesis). On the other hand, an individual with a high sensitivity to environmental stimuli would adapt its physiology to this kind of environment (match/mismatch hypothesis). Here, a moderate life time adversity would be most beneficial, as this would increase the resilience to subsequent mild or moderate stressors. Exposure to severe adversity would in all cases increase stress vulnerability and disease risk. Our data indicate that such different patterns (red vs. blue) might be a result of different genetic polymorphisms, where one allele favours low sensitivity (red), while the other allele favours a high sensitivity (blue). Therefore, each genotype can have beneficial as well as detrimental effects on stress vulnerability and stress-related disorders, depending on the degree of cumulative life time adversity.
Adverse experiences are an inevitable part of life and shape the physiology and behavioral responses of an individual. Stress exposure at various developmental time windows can improve resilience to stressful life events by preparing the individual for future challenges. However, chronic, uncontrollable or unpredictable stress exposure, as for example experienced by many during the worldwide Covid19 pandemic, can also increase disease vulnerability, significantly contributing to disease risk. While it is clear that the difference between stress vulnerability and resilience is grounded in the specific genetic and epigenetic background in concert with previous life experiences, the underlying mechanisms are still largely unknown. Consequently, it is not yet possible to predict with reasonable certainty whether an individual will respond to a future challenging situation with an adaptive or maladaptive stress response. The Schmidt Lab investigates the interplay of early experiences during different stages of development with genetic predisposition in shaping stress resilience. Our main focus lies on stress-related psychiatric disorders, like mood or anxiety disorders, stress-induced metabolic dysregulations leading to obesity and type-2 diabetes, as well as the interplay of psychiatric and metabolic disorders. In addition to gaining mechanistic insight into disease-relevant processes related to stress vulnerability, we also strive to carry out highly translational research. For this purpose, an important aspect of our work is the utilization of environmental, behavioral, dietary or pharmacological interventions, which can also be applied in humans.
